Coulomb’s Law
From grade 10 you should be familiar with positive and negative charges. Now, in grade 11, we learn how charged particles interact with one another.
Like charges repel, and unlike charges are attracted to one another. The Electrostatic force is the force which attracts/repels them.
Coulomb’s Law says that this force is proportional to both charges, and inversely proportional to
the square of the distance between them:
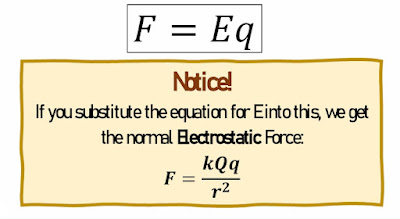
Electrostatic Force
We add a proportionality constant, 𝑘 = 9 × 109𝑁.𝑚2.𝐶−2, so that we can define the Electrostatic
force as:
Using this formula will give us the magnitude of the force, but to get the direction we must see if the
charges are like (repelling) or unlike (attracting).
It won’t make sense if you get a negative answer for
your magnitude, so double check your calculation!
Electric Field
The direction of the electric field at a point represents the direction of the force a positive charge
would experience (and the opposite direction for a negative charge).

















0 Comments