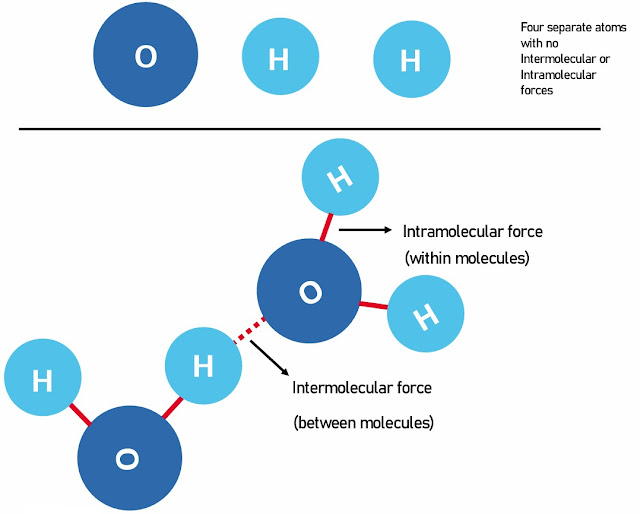
INTRAMOLECULAR AND INTERMOLECULAR FORCES
Intramolecular/Interatomic forces are forces that hold atoms together within a molecule, whereas Intermolecular forces are forces that exist between different molecules.
These are the 5 types of Intermolecular forces we will focus on:
- Dipole-dipole forces
- Mutually induced dipole forces (London forces)
- Dipole-induced dipole forces
- Ion-dipole forces
- Ion- induced-dipole forces
1. Dipole-dipole forces
When 2 Dipole molecules come into contact, the positive pole of one molecule will be
attracted to the negative pole of the other molecule and both molecules will be held together
by this attraction. These interactions are the strong intermolecular force of attraction.
Special Case– Hydrogen Bonding
Is a special dipole-dipole interaction that occurs SPECIFICALLY between a Hydrogen atom
bonded to either an Oxygen, Nitrogen or Fluorine atom. Hydrogen bonding is the strongest of
the dipole-dipole attractions and requires considerable energy to break these bonds which
results in compounds containing these bonds having exceptionally high boiling and melting
points
2. Mutually induced dipole forces (London forces):
These Intermolecular forces exist between all types of molecules but are the weakest of
forces. Molecules with more electrons will have the stronger London dispersion forces.
Breaking London dispersion forces do NOT require much energy which explains why Nonpolar covalent compounds will freeze at very low temperatures.
3. Dipole-induced dipole forces
This type of force occurs when a polar molecule induces a dipole in a non-polar molecule.
4. Ion-dipole forces
Forces between ions and polar molecules. An Ion is a charged atom, therefore will be
attracted to one of the poles of the polar molecule.
5. Ion- induced-dipole forces
Forces between ions and non-polar molecules, the ion induces a dipole leading to a weak
force that holds the compound together.
INTERMOLECULAR FORCE AND MOLECULAR MASS
The larger the molecule, the more electrons it
contains and therefore the molecule will be more
polar and thus the intermolecular forces
between the molecules would be stronger.
To
summaries, as the molecular mass increases so
does the intermolecular force. This can be
illustrated using alkanes which is shown in the
table below. The longer the alkane chain, i.e. the
greater the molecular mass, the higher the
boiling point of the alkane which indicates a
stronger intermolecular force.
THE EFFECT OF INTERMOLECULAR FORCES ON BOILING POINT, MELTING POINT, VAPOUR PRESSURE AND SOLUBILITY
Boiling point
Boiling point is the temperature at which the vapour pressure of a substance is equal to
the atmospheric pressure.
Melting point is the temperature at which the solid and liquid phases of a substance is at
equilibrium.
Vapour Pressure
Vapour pressure is the pressure exerted on a container when the vapour and liquid phases
of a substance is at equilibrium.
Solubility
Solubility is the ability of a certain substance to dissolve in another substance, for example
sugar dissolving in water.
Polar molecules are only soluble in polar solvents and non-polar molecules are only
soluble in non-polar solvents.























0 Comments